This post appeared in Italian on wired.it. This is the secon part of a (poor) English translation. First part is here
Not only the food is continuously monitored: also construction wood from the forests of
the region is controlled. In this case too the safety limits are much more
severe than that of the volcanic pozzolans often used in the construction
of houses and Italian schools.
 |
| Comparison between radiation Italy (Rome) and Japan (Tokyo and Saitama). It is possible to see how radioactive background is higher in Rome than Tokyo. In Rome the radiation environment is dominated by the peaks of Radon 222. The arrow indicates where the Cesium 137 (660 keV) peak should be located. |
An estimation of the amount of Cesium in food, wood or forage
requires a spectrometer capable of determining the energy of each gamma ray.
Since each isotope emits gamma rays of specific energies, it is possible to
determine the quantities of the various isotopes present.
Among the recent devices there is a
portable detector consisting of a crystal to stop the gamma (CsI) and a Silicon Photomultiplier (MMPC or
as they call them) to detect the energy measuring the light emitted in the
crystal. The simplicity of this type of tools is that they do not require high
voltages, are small as a pack of cigarettes and is used as a
USB device. The cost, however, is about 20 times that of a Geiger counter.
A spectrometer can count, for each decay, the energy of the rays
that strike it. In about an hour and then it is possible to obtain a spectrum which
describes the type and amount of environmental radiation. To improve the
statistics and better highlight the peaks is, however, advisable to wait a
while longer. The picture above shows the value measured at Rome in an
apartment on the fourth floor: it is 0.25microSv/hour (with peaks of 0.35).
In the figure above it is possible to see how
the spectrum in Rome (and in much of Italy) is dominated by radon 222, a noble
gas source to the high amount of environmental radiation. Usually the radon
comes from the soil and tuff, but in this case, since it is an apartment on the
fourth floor, is more likely to come from pozzolana used in the construction
materials . In figure are compared the spectra taken in Rome with those acquired in Japan. The value of Rome is higher (0.25microSv / h), followed by the
basement workshops of Tor Vergata (0.10 microSv / h, where, however, there is
much radon), Kokubunji (0.05), and the fourth floor in Wako (0035 microSv / h).
Note the almost total absence of radon in Japan. It is worth mentioning that
the environmental radiation at the onsen baths is higher due to the volcanic
nature of the sulphurous waters.
| Monitoring radiation in wood to be used for construction |
In the samples collected in the hot
spots, Cesium-137 is present in large quantities: this isotope decays into an
excited state of barium (emitting an electron and an antineutrino). The
de-excitation of barium emits a signal characteristic of this element: a gamma
ray energy of 660 keV. The process is similar to that of fluorescence, only
that in this case atomic electrons are excited. The return to the ground state
emits light (between 2 and 3 eV), e.g. electromagnetic radiation. The energy
levels in the nucleus are thousands of times more intense and therefore the
electromagnetic radiation emitted has an frequency and associated energy
thousands of times greater.
To the left of the peak there
is the so-called 'Compton edge', produced by gamma rays hitting an atomic
electron of the Cesium crystal in the detector and accelerating it with a
slightly lower energy (depending on the angle with which it is emitted). The
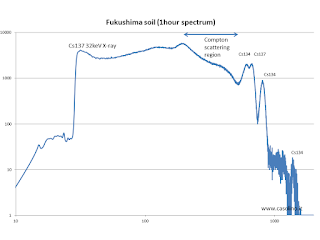
spectroscopic analysis of a particularly contaminated sample, taken on the side of a mountain road between the city
of Fukushima and the coast. This sample shows the presence of the isotope
cesium-134, which decays into barium with several peaks at 600, 790, 1400 and
1600 keV (the latter is out of range of the detector).
The cesium-134 has a decay time of
two years, therefore the presence of this isotope represents the
"signature" of the origin of the Fukushima power plant. In other cases, the
absence of cesium-134 was used to show how well mushrooms that had radioactivity
above the threshold of 100 Bq / kg were not contaminated by the panel, but
presumably from nuclear tests in the ‘60s.
 |
| Gamma spectrum from a pure Cs 137 source |
 |
| Spectrum of a sample of soil containing cesium 134 and 137 of Fukushima region |
The measures in the region of
Fukushima were extremely interesting, but equally important on a personal level
was the contact with the local population. Far from being disheartened, the people
in Tohokoku did not give up and have rebuilt many of the structures destroyed
by the tsunami. Although the plant has not resulted in deaths due to radiation
(morbidly sought by national and international journalists), many deaths are
due to poor management of the emergency in the first frantic days after the
earthquake. Others are due to suicides after resettlement. The inhabitants of
the regions closer to the center have been forcefully moved away and are now
rebuilding the social fabric elsewhere, so sometimes it is difficult to return
to their town of origin, even if it were decontaminated. The most relevant problem
is economic: the damage to the primary sector and tourism are visible to all
and will require years to get back to normal.
2. end First part is here